12-02-2025
Streamlining the Process of Identifying KOLs: The konectar Advantage

Key Opinion Leaders (KOLs) are influential experts in healthcare. These individuals are valued for their deep expertise, research contributions, and ability to guide clinical practices.
Their insights bridge the gap between scientific innovation and real-world medical applications, playing a crucial role in patient care and treatment outcomes.
In this article:
- The Importance of Streamlining KOL Identification
- The konectar Advantage
- Key Benefits of konectar
- Key Takeaway
- FAQs
The Importance of Streamlining KOL Identification
The ability to identify and engage with the right KOLs is of strategic importance for pharma companies of today. Streamlining this process ensures that organizations can quickly connect with experts with the relevant knowledge, credibility, and influence to drive a meaningful change in clinical practices and treatment adoption.
An efficient KOL identification process eliminates redundancies, reduces operational delays, and provides actionable insights to the right team at the right time. This accelerates drug development and enhances market access, fostering stronger collaboration between pharmaceutical teams and influential medical professionals.
In a highly competitive environment, quickly and accurately identifying KOLs can distinguish between a successful launch and a missed opportunity.
Additionally, streamlining the identification process improves internal resource allocation, allowing medical affairs teams to focus on meaningful engagement rather than navigating fragmented data and outdated methods.
Why Rapid & Accurate KOL Identification Matters
Healthcare and pharmaceutical sectors are characterized by extremely tight timelines, high regulatory standards, and significant financial investments. In such an environment, rapid and accurate KOL identification is critical in ensuring that the organization’s objectives are met efficiently.
Accurate identification ensures that the right experts are engaged from the outset, whether for designing clinical trials, navigating regulatory approvals, or promoting new therapies.
Early engagement with credible KOLs enhances the reliability of clinical outcomes, improves the adoption of evidence-based practices, and builds trust within the medical community.
Simultaneously, it eliminates delays or inaccuracies in KOL identification, which can lead to costly missteps. Collaborating with less relevant or underqualified individuals can lead to wasted resources, ineffective engagement strategies, and delayed timelines.
Additionally, as pharmaceutical companies expand globally, the ability to quickly identify region-specific KOLs who understand local healthcare landscapes becomes essential.
In short, rapid and accurate KOL identification is not about speed but ensuring that every engagement delivers maximum impact.
Common Challenges in KOL Identification
Despite its significance, KOL identification remains a complex and challenging process, requiring pharma companies to partner with KOL identification companies. Inefficiencies, fragmented data, and subjective decision-making frequently mar traditional approaches.
Data Silos
One of the biggest obstacles in KOL identification is data fragmentation across multiple sources. Relevant information about potential KOLs, such as publication records, clinical trial participation, conference presentations, and professional affiliations, is often scattered across disparate platforms.
Medical Affairs teams must spend valuable time manually collating and cross-referencing this data, which can lead to inefficiencies and delays.
Manual Processes Leading to Delays
For many organizations, KOL identification still relies heavily on manual processes, including spreadsheet analysis and database searches. These methods are time-consuming and prone to human error.
As a result, medical teams may overlook valuable KOLs or waste time pursuing less relevant individuals. Such delays can significantly impact clinical trial timelines and product launches.
Difficulty Assessing Real Influence and Relevance
Not all KOLs with publications or clinical trial involvement have the same influence. Traditional evaluation criteria often focus on surface-level metrics, such as the number of published papers, while overlooking nuanced factors, such as peer recognition, leadership roles, and influence within specific therapeutic areas.
This lack of granularity can lead to ineffective engagements and missed opportunities for collaboration.
Overcoming these challenges requires a combination of advanced tools, data analytics, and a strategic approach to KOL engagement.
The konectar Advantage
konectar transforms how life sciences teams connect with Key Opinion Leaders (KOLs), delivering AI-driven insights with speed and precision. Its intelligent platform empowers organizations to identify, profile, and engage the right experts effortlessly, eliminating the complexities of traditional KOL management.
Smarter Insights, Stronger Connections
With access to vast industry intelligence, konectar ensures that companies stay ahead by identifying the most relevant experts across therapeutic areas, geographies, and specialties. Its dynamic approach enhances decision-making, making every engagement more strategic and impactful.
Tailored Ranking & Targeting
konectar’s advanced capabilities allow organizations to prioritize experts based on relevance and influence. With intelligent ranking and segmentation, teams can focus on the right KOLs—those who align perfectly with their objectives.
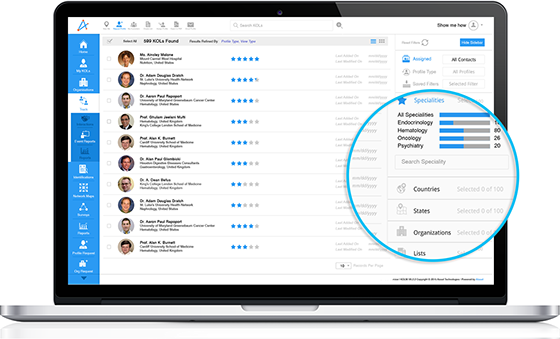
Effortless Search & Discovery
Designed for precision, konectar streamlines expert discovery with intuitive search and filtering tools, helping teams navigate vast networks with ease and accuracy.
Redefining KOL Engagement
By offering deep professional insights and seamless profile management, konectar bridges the gap between identification and collaboration, enabling stronger, data-driven relationships.
Geographical Filters
The search filter can be refined by region, country, or city, which is vital for KOL identification.
These advanced search capabilities help teams bypass irrelevant profiles and focus on experts aligned with their campaign or project goals.
KOL Profile Management
One of konectar's most impressive features is its KOL profile management tool, which ensures seamless workflows across cross-functional teams.
Profile Enrichment
Each KOL profile aggregates key details, such as academic and clinical background, professional network, and collaborations, into a centralized, easy-to-access format.
Network Mapping
Visual representations of professional networks reveal how KOLs are connected to other influencers and organizations.
For pharmaceutical teams, medical affairs professionals, and healthcare marketers, konectar is an end-to-end KOL management platform bridging the gap between identification, collaboration, and engagement.
Key Benefits of konectar
konectar’s robust capabilities empower medical affairs teams to maximize their outreach efforts while minimizing inefficiencies.
Time and Cost Efficiency
One of the most significant advantages of konectar lies in its ability to streamline processes and reduce operational costs associated with traditional KOL identification methods.
Automation of Manual Processes
Instead of spending hours sifting through spreadsheets, scientific journals, and disparate databases, konectar automates data aggregation and analysis.
This significantly reduces the time spent on research, allowing teams to focus on engagement rather than data collection.
Reduced Resource Wastage
The platform minimizes duplication of effort across teams by consolidating all KOL data in one central hub, ensuring every team member operates from the same source of information, be it a local digital opinion leader or a global key opinion leader.
Timely Insights
Real-time dashboards and automated analytics provide immediate access to actionable insights, speeding up decision-making processes for clinical trial recruitment, product launches, or regulatory approvals.
Cost Savings Through Precision
By identifying the most relevant KOLs quickly and accurately, konectar ensures that outreach efforts are focused on experts who can deliver the most impact, reducing unnecessary expenditures on ineffective partnerships.
In essence, konectar transforms time-consuming, resource-heavy identification processes into lean, cost-efficient operations, delivering maximum value with minimum waste.
Accuracy and Relevance
Within the world of healthcare, precision isn’t optional. It’s essential. konectar excels at providing accurate, data-driven insights to ensure that the most relevant KOLs are identified, engaged, and nurtured.
Data-Driven Scoring and Ranking
The platform uses customizable scoring algorithms to rank KOLs based on metrics like clinical trial experience, regional influence, and speaking engagements.
Objective Evaluation
By eliminating biases associated with manual identification methods, konectar objectively assesses each KOL’s influence and relevance.
Customized Filters
Teams can refine searches based on therapeutic areas such as geography, publications, and metrics, ensuring they engage with the right experts for the ridge projects.
With konectar, pharmaceutical teams can make informed decisions backed by reliable data, reducing the risk of misaligned partnerships and ineffective engagements.
Scalable and Flexible
The dynamic nature of pharmaceutical projects, ranging from niche clinical trials to global product launches, requires tools that can adapt and grow with organizational needs.
konectar offers unparalleled scalability and flexibility to meet these demands.
Adaptable to Project Scope
Whether teams need to identify a handful of niche experts for a regional project or thousands of KOLs for a global campaign, konectar scales seamlessly to match project size and complexity.
Personalized Dashboards
The platform’s dashboards can be tailored to display the most relevant metrics for each team: Medical Affairs, Marketing, or Regulatory Affairs.
Therapeutic Area Flexibility
From oncology to neurology, konectar is designed to handle data across diverse therapeutic areas without requiring separate systems or tools.
Integration with Existing Systems
konectar can integrate effortlessly with CRM systems, HCP management tools, and data analytics platforms, ensuring smooth workflows without extensive modifications.
Discover the full potential of your KOL strategy with konectar.
Precision, scalability, and efficiency define success in pharmaceutical engagement. This is where konectar emerges as an ultimate solution for streamlining KOL identification, profiling, and engagement.
Discover the right experts, save time and resources, gain actionable insights, scale seamlessly, and more, all at your fingertips.
Request a demo today and transform your KOL strategy into a competitive advantage that drives results.
Key Takeaway
For the rapidly evolving landscape, konectar stands out as an AI-powered solution that redefines KOL identification, profiling, and engagement.
konectar is more than a platform. It’s a strategic partner in building meaningful, data-driven relationships with the right KOLs to drive impactful outcomes.
FAQs
- What is the importance of streamlining KOL identification?
Streamlining KOL identification is crucial to quickly and accurately connect with the right experts. It eliminates redundancies, reduces delays, and ensures data-driven decision-making.
- What are some of the common challenges in KOL identification?
Common challenges include data silos, manual processes leading to delay, and difficulty assessing the actual influence and relevance of potential KOLs.
- What types of data does konectar aggregate?
konectar aggregates scientific publications, clinical trial data, conference participation, professional affiliations, and more.
- How often is KOL data updated?
KOL data in konectar is updated in real-time to ensure its accuracy and relevancy.